GLYCINE ON IRON OXYHYDROXYDES: IR SPECTROSCOPY
Сучасне матеріало- та товарознавство :: 1. Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
 GLYCINE ON IRON OXYHYDROXYDES: IR SPECTROSCOPY
GLYCINE ON IRON OXYHYDROXYDES: IR SPECTROSCOPY
Yu. E. Sakhno
Laboratoire de Réactivité de Surface
UMR 7197, UPMC, case courrier 178
4 Place Jussieu 75252 Paris CEDEX 05
GLYCINE ON IRON OXYHYDROXYDES: IR SPECTROSCOPY
The adsorption of amino acids on inorganic oxide surfaces has been the object of many experimental studies, which were recently reviewed [ ].
We have vibrational spectroscopy studied the behavior upon thermal activation of glycine adsorbed on three well-characterized Fe3+ oxide nanoparticle phases, maghemite, hematite and akaganeite. The behavior of the adsorbed molecules and of the nanoparticles surfaces were followed by four main experimental techniques, TGA-DTA, XPS, vibrational spectroscopy (IR), and mass spectrometry. Glycine polymerizes by peptide bond formation in the 180-190°C temperature range, i.e. somewhat higher than on previously studied oxides such as silica or alumina, giving mostly short linear peptides. At slightly higher temperatures, under inert atmosphere, the iron oxyhydroxides act as stoechiometric oxidants and cause oxidative degradation of the peptides formed in the previous step, while they are reduced to FeO; under air, dioxygen causes reoxidation of the nanoparticle surfaces so that the overall effect is a catalytic oxidation by O2. While the direct formation of linear peptides may be beneficial to prebiotic complexity growth, the redox reactivity of the supports limits the temperature stability ranged of the oligopeptides. Infrared spectroscopy:
a) Diffuse reflectance mode ( RFT): Spectra were recorded using arer FSV spectrometer (cm−1 resolution, 256 scans/spectrum, MCT detector). About 40 mg of powdered sample was placed inside a heated crucible located in a Thermo Spectra-Tech high temperature cell equipped with two ZnSe windows and under Ar flow. The reference spectrum was recorded with KBr (Fluka, purity>99.5%). b) Transmission mode: Infrared spectra of the samples were collected using rerFS (cm−1 resolution, 256 scans/spectrum, DTGS detector) in the transmission mode. The lab-made cell used, equipped with CaF2 windows (see above) was permanently attached to a conventional vacuum line (residual pressure. 1×10-4 mbar), allowing thermal treatments and desorption/adsorption experiments to be carried out in situ. The optical thickness of the oxyhydroxyde pellets was in the 4 to 6 mg/cm2 range.
Adsorption of Glycine from liquid phase. The spectra of Gly/Mgh, Gly/Ht and Gly/Aka are shown in Figures 1, 2, 3. Table 1 proposes assignments for the the main adsorption bands observed in the 1000-1800 cm-1 range (in which the iron oxides supports are mostly transparent), on the basis of known band positions in bulk -glycine [ ] Several bands in the supported Gly systems have positions very similar to bulk glycine.

Figure 1: DRIFT spectra of Maghemite (a) and Gly/Mgh Figure 2: DRIFT spectra of Hematite
(b) corrected by KBr spectrum (a) and Gly/Ht (b) corrected by KBr spectrum

Figure 3: DRIFT spectra of Akaganeite (a) and Figure 4: Difference DRIFT spectra of Gly/Mgh
Gly/Aka (b) corrected by KBr spectrum (corrected for spectrum at 30 °C) under in situ thermal activation at a. 50 °C,
b. 70 °C, c. 100 °C, d. 150 °C, e. 190 °C, f. 200 °C, g. 220 °C, h. 250 °C.
This indicates that the glycine molecules are in a weak interaction with the surface, such as hydrogenbonding (in bulk glycine too, the individual molecules are involved in a network of H-bonds with their neighbors[6j]), but not establishing strong bonds such as coordination to surface ions. An exception is observed for the carboxylate group vibration in Gly/Mgh, which shifts significantly, from 1412 cm-1 (in bulk glycine) to 1380 cm-1. This shift could be interpreted as the consequence of a strong interaction between maghemite surface and glycine, possibly through the formation of coordinative bonds between the carboxylate moiety and surface Fe3+ ions. It is not observed for Gly/SiO2 [ ] - and neither is it for the other two Fe oxyhydroxide supports (Table 1).
Table 1 : DRIFT spectroscopy - main vibrational bands of bulk glycine and adsorbed glycine samples- n.o. = not observed

We have next tried to determine how the vibrational spectra of supported glycine samples evolve with thermal activation. We present our data as difference spectra, i.e. the spectrum at the indicated temperature has been corrected by subtracting the spectrum at 30 °C. For Gly/Mgh (Figure 4), in situ thermal activation first causes an increase of a signal centered at 1697 cm-1, which is most manifest at 150°C. This might be due to the appearance of an amide I vibrational band. However, if that were the case a positive signal corresponding to an amide II band should appear at the same time around 1580 cm-1, and such a signal is not observed. Starting at 120°C, negative signals are visible as well in the difference spectra and become more and more prominent up to 250°C. Their positions correspond to the most intense bands of glycine, which means that we are mostly witnessing the progressive elimination of glycine. Water loss may also contribute to the negative peak in the 1630 cm-1 region. However this negative peak does not appear before 120°C. Therefore, the most likely explanation is that water elimination is already largely completed at 30°C under vacuum.
At higher temperatures, the carbonyl band decreases significantly whereas the component at 1717 cm-1 decreases to a lesser extent.
On hematite, the thermal behavior is different (Fig 5).
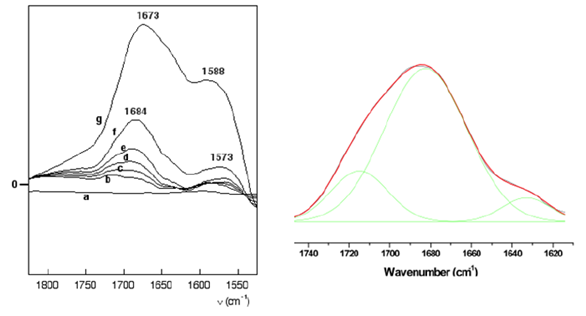
Figure 5: left, difference DRIFT spectra of Gly/Ht (corrected for spectrum at 30°C) under in situ thermal activation at a. 50°C, b. 70°C, c. 100 °C, d. 150°C, e. 200°C, f. 220°C, g. 250°C. Right, decomposition of the amide I band of Gly/Ht 150°C.
Up to 220 °C two components increase simultaneously, with maxima around 1680 and 1580 cm-1. They could correspond to the amide I and amide II bands, confirming the formation of peptides. Between 150 and 220°C, the amide I band consists of two components at 1680 cm-1 and 1630 cm-1. Possible reasons for the existence of these two components will be considered in the discussion. At the highest temperatures, the band maximum shifts to 1673 cm-1, which correspond to the amide link of the cyclic dimer DKP [ ]. The presence of DKP is also suggested by a band at 1469 to 1478 cm-1 in the substracted spectra (not shown) which could be the breathing mode of the DKP cycle[4].
A component at around 1715 cm-1 is necessary for a correct fit of the high-energy side of the amide I band. None of the canonical peptide structures gives an amide I band at such a high wavenumber, and thus it could be due to degradation products. Indeed, for Gly/SiO2, Lambert et al [3] have observed a band at 1716 cm-1 after activation at relatively high temperatures; in this study, it was probably a product of DKP thermal evolution and was not investigated further. After heating to 250°C, the proportion of the component at 1680 cm-1 increases significantly compared to the band at 1705 cm-1, which would mean that most of the glycine molecules that had not yet transformed at 150°C condense to form peptides (including DKP) rather than other degradation products. On akaganeite, thermal activation induces the progressive increase of amide I and II bands (Figure 6).
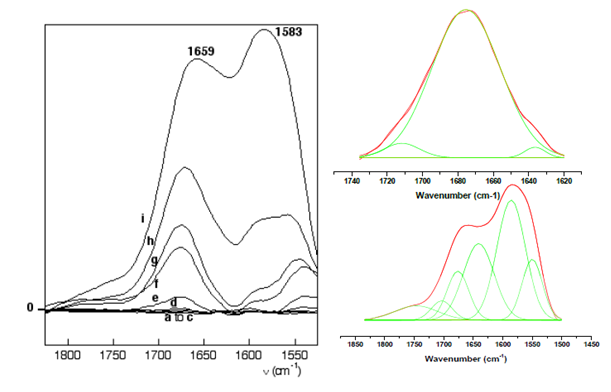
Figure 6 : Left, difference DRIFT spectra of Gly/Aka (corrected for spectrum at 30°C) under in situ thermal activation at a. 50°C, b. 70°C, c. 100 °C, d. 150°C, e. 170°C, f. 190°C, g. 200°C, h. 220°C, i. 250°C. Right, decomposition of the amide I band of Gly/Aka at 150°C. Bottom, decomposition of the amide I and II bands of Gly/Aka at 250°C.
At 150°C, the amide I band is centered at 1659 cm-1 and can be fitted with three components at 1635 cm-1, 1675 cm-1 and 1691 cm-1 (the introduction of a fourth band does not improve the quality of the fit). No band is observed around 1710 cm-1 until 250°C, indicating the stability of glycine-derived peptides on akaganeite. The presence of DKP is suggested by a band at 1460 cm-1,.in the same way as on maghemite. At 250°C (Figure 6), the components of the amide I vibrational band are centered at 1641 cm-1, 1677 cm-1 and 1700 cm-1. A contribution at 1749 cm-1 probably corresponds to the CO vibration of COOH groups in products of degradation, as on maghemite, but it remains a minor component. The shift of vibrational band could be due to an amide band in another environment as observed in the case of hematite, but also due to the decrease of the proportion of DKP (1670 cm-1). Nevertheless, the position of the amide I band shifts from 1660 cm-1 at 150°C to 1600 cm-1 at 200°C. After thermal activation at 200°C, the intensity in the 1750-1500 cm-1 region decreases as a consequence of water and probably glycine desorption. It should be added that in this particular case, intensity changes are also observed in the 3000- 3600 cm-1 region, corresponding to OH stretching mode (not shown). They correspond to the thermal modifications of structural OH in akaganeite. In particular, a significant overall decrease is observed starting from 220°C, when the condensation of OH groups that accompanies the transition to hematite is already underway.
Laboratoire de Réactivité de Surface
UMR 7197, UPMC, case courrier 178
4 Place Jussieu 75252 Paris CEDEX 05
GLYCINE ON IRON OXYHYDROXYDES: IR SPECTROSCOPY
The adsorption of amino acids on inorganic oxide surfaces has been the object of many experimental studies, which were recently reviewed [ ].
We have vibrational spectroscopy studied the behavior upon thermal activation of glycine adsorbed on three well-characterized Fe3+ oxide nanoparticle phases, maghemite, hematite and akaganeite. The behavior of the adsorbed molecules and of the nanoparticles surfaces were followed by four main experimental techniques, TGA-DTA, XPS, vibrational spectroscopy (IR), and mass spectrometry. Glycine polymerizes by peptide bond formation in the 180-190°C temperature range, i.e. somewhat higher than on previously studied oxides such as silica or alumina, giving mostly short linear peptides. At slightly higher temperatures, under inert atmosphere, the iron oxyhydroxides act as stoechiometric oxidants and cause oxidative degradation of the peptides formed in the previous step, while they are reduced to FeO; under air, dioxygen causes reoxidation of the nanoparticle surfaces so that the overall effect is a catalytic oxidation by O2. While the direct formation of linear peptides may be beneficial to prebiotic complexity growth, the redox reactivity of the supports limits the temperature stability ranged of the oligopeptides. Infrared spectroscopy:
a) Diffuse reflectance mode ( RFT): Spectra were recorded using arer FSV spectrometer (cm−1 resolution, 256 scans/spectrum, MCT detector). About 40 mg of powdered sample was placed inside a heated crucible located in a Thermo Spectra-Tech high temperature cell equipped with two ZnSe windows and under Ar flow. The reference spectrum was recorded with KBr (Fluka, purity>99.5%). b) Transmission mode: Infrared spectra of the samples were collected using rerFS (cm−1 resolution, 256 scans/spectrum, DTGS detector) in the transmission mode. The lab-made cell used, equipped with CaF2 windows (see above) was permanently attached to a conventional vacuum line (residual pressure. 1×10-4 mbar), allowing thermal treatments and desorption/adsorption experiments to be carried out in situ. The optical thickness of the oxyhydroxyde pellets was in the 4 to 6 mg/cm2 range.
Adsorption of Glycine from liquid phase. The spectra of Gly/Mgh, Gly/Ht and Gly/Aka are shown in Figures 1, 2, 3. Table 1 proposes assignments for the the main adsorption bands observed in the 1000-1800 cm-1 range (in which the iron oxides supports are mostly transparent), on the basis of known band positions in bulk -glycine [ ] Several bands in the supported Gly systems have positions very similar to bulk glycine.

Figure 1: DRIFT spectra of Maghemite (a) and Gly/Mgh Figure 2: DRIFT spectra of Hematite
(b) corrected by KBr spectrum (a) and Gly/Ht (b) corrected by KBr spectrum

Figure 3: DRIFT spectra of Akaganeite (a) and Figure 4: Difference DRIFT spectra of Gly/Mgh
Gly/Aka (b) corrected by KBr spectrum (corrected for spectrum at 30 °C) under in situ thermal activation at a. 50 °C,
b. 70 °C, c. 100 °C, d. 150 °C, e. 190 °C, f. 200 °C, g. 220 °C, h. 250 °C.
This indicates that the glycine molecules are in a weak interaction with the surface, such as hydrogenbonding (in bulk glycine too, the individual molecules are involved in a network of H-bonds with their neighbors[6j]), but not establishing strong bonds such as coordination to surface ions. An exception is observed for the carboxylate group vibration in Gly/Mgh, which shifts significantly, from 1412 cm-1 (in bulk glycine) to 1380 cm-1. This shift could be interpreted as the consequence of a strong interaction between maghemite surface and glycine, possibly through the formation of coordinative bonds between the carboxylate moiety and surface Fe3+ ions. It is not observed for Gly/SiO2 [ ] - and neither is it for the other two Fe oxyhydroxide supports (Table 1).
Table 1 : DRIFT spectroscopy - main vibrational bands of bulk glycine and adsorbed glycine samples- n.o. = not observed

We have next tried to determine how the vibrational spectra of supported glycine samples evolve with thermal activation. We present our data as difference spectra, i.e. the spectrum at the indicated temperature has been corrected by subtracting the spectrum at 30 °C. For Gly/Mgh (Figure 4), in situ thermal activation first causes an increase of a signal centered at 1697 cm-1, which is most manifest at 150°C. This might be due to the appearance of an amide I vibrational band. However, if that were the case a positive signal corresponding to an amide II band should appear at the same time around 1580 cm-1, and such a signal is not observed. Starting at 120°C, negative signals are visible as well in the difference spectra and become more and more prominent up to 250°C. Their positions correspond to the most intense bands of glycine, which means that we are mostly witnessing the progressive elimination of glycine. Water loss may also contribute to the negative peak in the 1630 cm-1 region. However this negative peak does not appear before 120°C. Therefore, the most likely explanation is that water elimination is already largely completed at 30°C under vacuum.
At higher temperatures, the carbonyl band decreases significantly whereas the component at 1717 cm-1 decreases to a lesser extent.
On hematite, the thermal behavior is different (Fig 5).

Figure 5: left, difference DRIFT spectra of Gly/Ht (corrected for spectrum at 30°C) under in situ thermal activation at a. 50°C, b. 70°C, c. 100 °C, d. 150°C, e. 200°C, f. 220°C, g. 250°C. Right, decomposition of the amide I band of Gly/Ht 150°C.
Up to 220 °C two components increase simultaneously, with maxima around 1680 and 1580 cm-1. They could correspond to the amide I and amide II bands, confirming the formation of peptides. Between 150 and 220°C, the amide I band consists of two components at 1680 cm-1 and 1630 cm-1. Possible reasons for the existence of these two components will be considered in the discussion. At the highest temperatures, the band maximum shifts to 1673 cm-1, which correspond to the amide link of the cyclic dimer DKP [ ]. The presence of DKP is also suggested by a band at 1469 to 1478 cm-1 in the substracted spectra (not shown) which could be the breathing mode of the DKP cycle[4].
A component at around 1715 cm-1 is necessary for a correct fit of the high-energy side of the amide I band. None of the canonical peptide structures gives an amide I band at such a high wavenumber, and thus it could be due to degradation products. Indeed, for Gly/SiO2, Lambert et al [3] have observed a band at 1716 cm-1 after activation at relatively high temperatures; in this study, it was probably a product of DKP thermal evolution and was not investigated further. After heating to 250°C, the proportion of the component at 1680 cm-1 increases significantly compared to the band at 1705 cm-1, which would mean that most of the glycine molecules that had not yet transformed at 150°C condense to form peptides (including DKP) rather than other degradation products. On akaganeite, thermal activation induces the progressive increase of amide I and II bands (Figure 6).

Figure 6 : Left, difference DRIFT spectra of Gly/Aka (corrected for spectrum at 30°C) under in situ thermal activation at a. 50°C, b. 70°C, c. 100 °C, d. 150°C, e. 170°C, f. 190°C, g. 200°C, h. 220°C, i. 250°C. Right, decomposition of the amide I band of Gly/Aka at 150°C. Bottom, decomposition of the amide I and II bands of Gly/Aka at 250°C.
At 150°C, the amide I band is centered at 1659 cm-1 and can be fitted with three components at 1635 cm-1, 1675 cm-1 and 1691 cm-1 (the introduction of a fourth band does not improve the quality of the fit). No band is observed around 1710 cm-1 until 250°C, indicating the stability of glycine-derived peptides on akaganeite. The presence of DKP is suggested by a band at 1460 cm-1,.in the same way as on maghemite. At 250°C (Figure 6), the components of the amide I vibrational band are centered at 1641 cm-1, 1677 cm-1 and 1700 cm-1. A contribution at 1749 cm-1 probably corresponds to the CO vibration of COOH groups in products of degradation, as on maghemite, but it remains a minor component. The shift of vibrational band could be due to an amide band in another environment as observed in the case of hematite, but also due to the decrease of the proportion of DKP (1670 cm-1). Nevertheless, the position of the amide I band shifts from 1660 cm-1 at 150°C to 1600 cm-1 at 200°C. After thermal activation at 200°C, the intensity in the 1750-1500 cm-1 region decreases as a consequence of water and probably glycine desorption. It should be added that in this particular case, intensity changes are also observed in the 3000- 3600 cm-1 region, corresponding to OH stretching mode (not shown). They correspond to the thermal modifications of structural OH in akaganeite. In particular, a significant overall decrease is observed starting from 220°C, when the condensation of OH groups that accompanies the transition to hematite is already underway.
Сучасне матеріало- та товарознавство :: 1. Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
Права доступу до цього форуму
Ви не можете відповідати на теми у цьому форумі